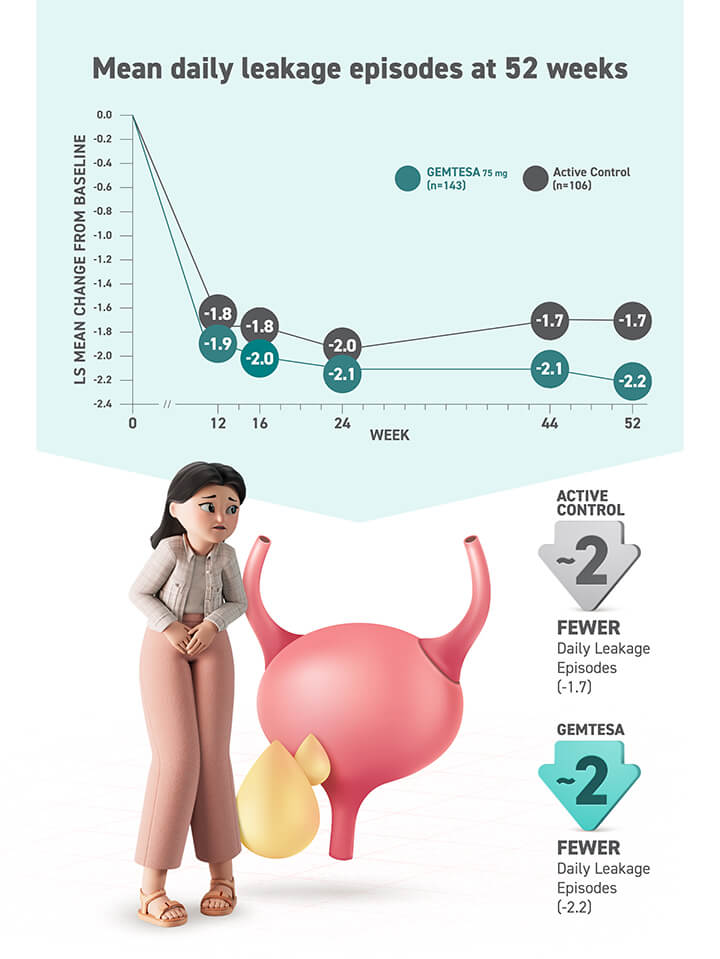
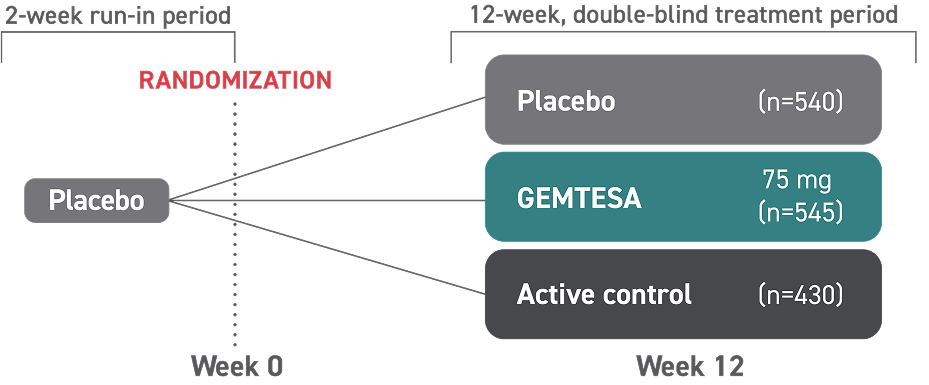
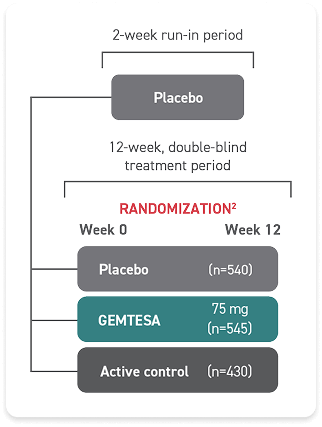
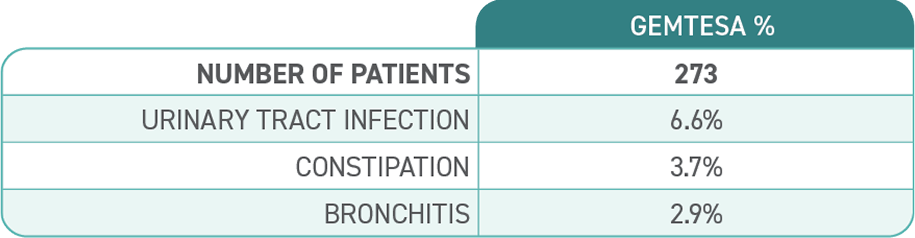
Diagnosis is a key first step toward treating OAB1

Ask your patients about their OAB symptoms and note if they
Feel the urge to urinate regardless of how
recently they last went to the bathroom
Have to urinate 8+ times per day, even after
only drinking a small amount
Travel with extra pads or clothing in case of
an accident
Avoid scenarios where a bathroom may not
be readily available
When you proactively screen for all urinary symptoms, you can identify male patients struggling with OAB1
Male patients may be reluctant to share how OAB symptoms burden their lives. Make complete urinary health screening part of routine care for all patients.2-4
AUA/SUFU Guideline
When evaluating patients, see if the OAB symptoms HIT1:
H
I
T
History
Review medical history with comprehensive assessment of bladder symptoms
Inspect
Perform a thorough physical examination
Test
Order a urinalysis to exclude microhematuria and infection
AUA=American Urological Association; BPH=benign prostatic hyperplasia; OAB=overactive bladder; SUFU=Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction.