INDICATIONS AND USAGE
GEMTESA® is a beta-3 adrenergic agonist indicated for the treatment of:
- overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency in adults.
- overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency in adult males on pharmacological therapy for benign prostatic hyperplasia (BPH).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
GEMTESA is contraindicated in patients with known hypersensitivity to vibegron or any components of GEMTESA. Hypersensitivity reactions, such as angioedema, have occurred.
WARNINGS AND PRECAUTIONS
Urinary Retention
Urinary retention has been reported in patients taking GEMTESA. The risk of urinary retention may be increased in patients with bladder outlet obstruction and also in patients taking muscarinic antagonist medications for the treatment of OAB. Monitor patients for signs and symptoms of urinary retention, particularly in patients with bladder outlet obstruction or patients taking muscarinic antagonist medications for the treatment of OAB. Discontinue GEMTESA in patients who develop urinary retention.
Angioedema
Angioedema of the face and/or larynx has been reported with GEMTESA. Angioedema has been reported to occur hours after the first dose or after multiple doses. Angioedema, associated with upper airway swelling, may be life-threatening. If involvement of the tongue, hypopharynx, or larynx occurs, immediately discontinue GEMTESA and provide appropriate therapy and/or measures necessary to ensure a patent airway.
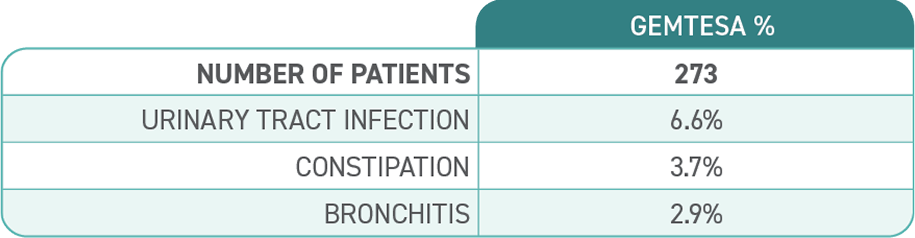
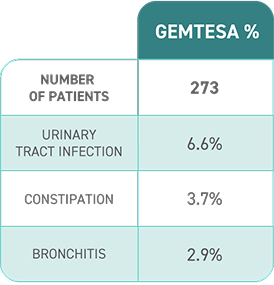
ADVERSE REACTIONS
Most common adverse reactions (≥2%) reported with GEMTESA were headache, urinary tract infection, nasopharyngitis, diarrhea, nausea, and upper respiratory tract infection.