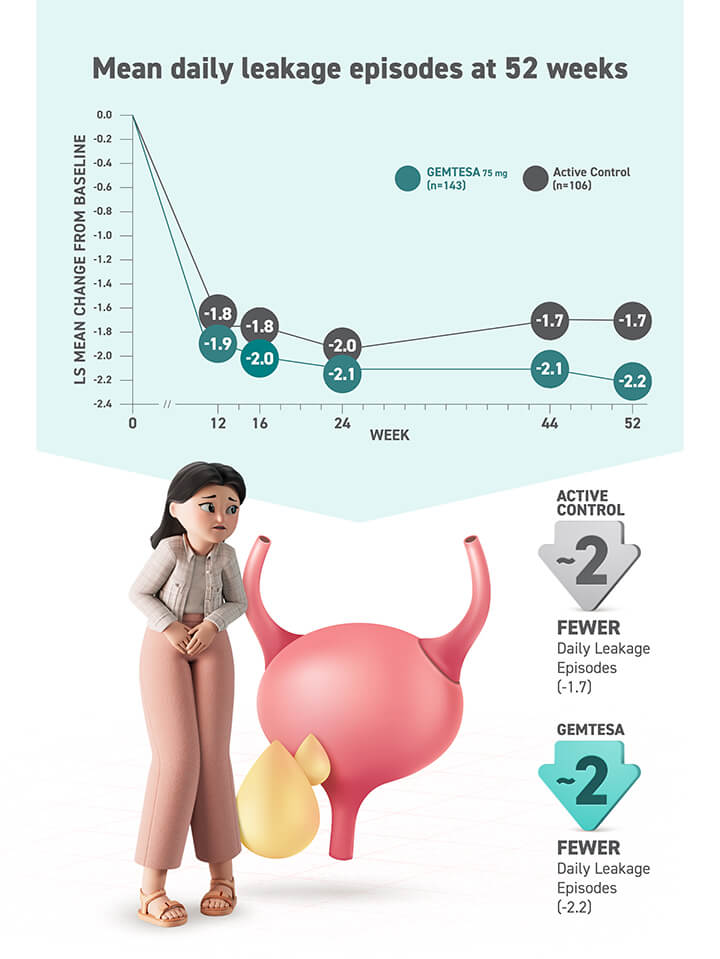
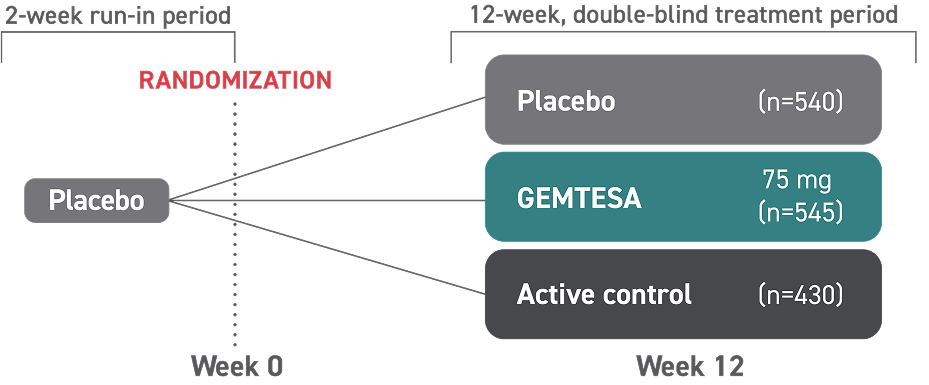
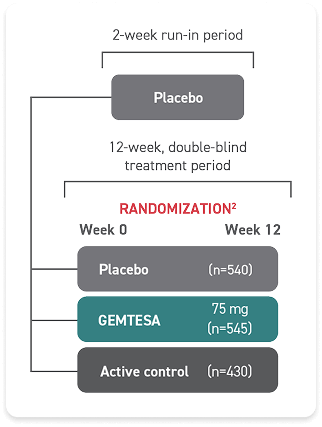
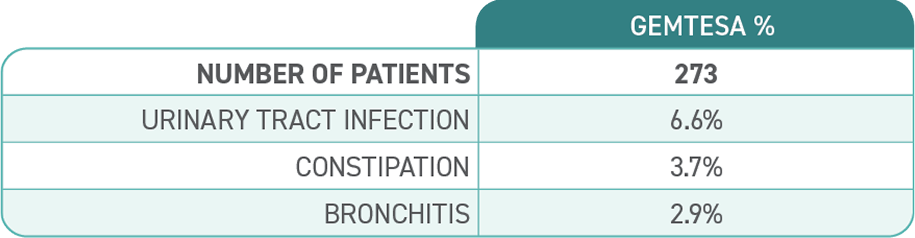
Proven safety and tolerability

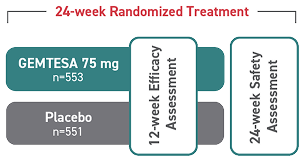
Adverse events compared to placebo at 12 weeks1,2
98.3%
OF PATIENTS IN THE GEMTESA ARM COMPLETED THE 12-WEEK STUDY WITHOUT DISCONTINUING TREATMENT DUE TO AEs3
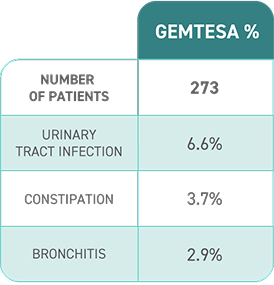
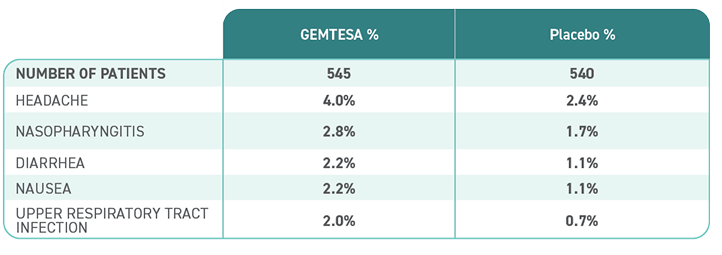
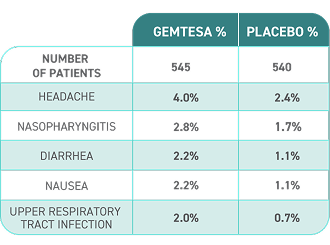
Reported in ≥2% of patients treated with GEMTESA 75 mg for up to 12 weeks and exceeding control arm.1,4
Reported in ≥2% of patients treated with GEMTESA 75 mg for up to 12 weeks and exceeding control arm.1,4
The first and only β3-agonist with no blood pressure warning in its label1*
- In a 24-week study of OAB in men being treated for BPH, rates of hypertension were 9.0% with GEMTESA (n=553) vs 8.3% with placebo (n=551)1,5
BY AGE 45, NEARLY ~50% OF WOMEN AND MEN HAVE HYPERTENSION6†
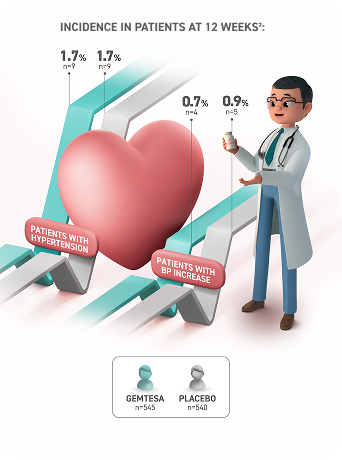
NO CLINICALLY SIGNIFICANT IMPACT ON HYPERTENSION OR BLOOD PRESSURE1,2,5‡
The incidence of hypertension and increased blood pressure at 12 weeks was consistent with results seen at 52 weeks.2,3,7
* In a 12-week pivotal study, hypertension rates for OAB patients taking GEMTESA (n=545) were 1.7% vs 1.7% with placebo (n=540). Increased BP rates were 0.7% with GEMTESA vs 0.9% with placebo.2
† Based on NHANES data conducted from 2011 to 2014, including 9623 participants.6
‡ In a 4-week, randomized, placebo-controlled, ambulatory BP study in OAB patients (n=200), GEMTESA 75 mg daily was not associated with clinically significant changes in BP. Mean age 59 years; 75% female. At baseline: 35% of subjects had preexisting hypertension; 29% were taking at least 1 concomitant antihypertensive medication.1
Annually, patients with OAB take an average of 12-14 medications, many of which are metabolized by the CYP2D6 pathway3,8-10*
Common comorbidities in patients with OAB include3,8-10:
Hypertension†
Depression
Diabetes
* Data from 2 studies. The first study examined patients (65.4% were ≥75 years old) with OAB who had concurrent medical conditions and received concomitant medications (n=415). The second study analyzed the number of medications taken by patients ≥65 years old during a more than 1-year period (n=1,801,457).3,9
† Defined as an average increase of SBP ≥20 mm Hg or DBP ≥10 mm Hg on 3 assessments at two consecutive visits, or the initiation or increase in dose of antihypertensive medications at any visit, in hypertensive patients.1
† Defined as an average SBP ≥140 mm Hg or diastolic BP (DBP) ≥90 mm Hg on 3 assessments at two consecutive visits, in non-hypertensive patients.1
GEMTESA does not affect the efficacy or safety of medications metabolized by the CYP2D6 pathway1,8
The first and only β3-agonist with no CYP2D6 drug interactions1
79% OF PATIENTS TAKING AN OAB MEDICATION ALSO TAKE AN AGENT METABOLIZED BY CYP2D63*
Concomitant use of GEMTESA increases digoxin maximal concentrations (Cmax) and systemic exposure as assessed by area under the concentration-time curve (AUC). Serum digoxin concentrations should be monitored before initiating and during therapy with GEMTESA and used for titration of the digoxin dose to obtain the desired clinical effect. Continue monitoring digoxin concentrations upon discontinuation of GEMTESA and adjust digoxin dose as needed.1
Common CYP2D6-metabolized medications include10-13:
- BETA-BLOCKERS: metoprolol, carvedilol, propranolol
- SSRIs/SNRIs: fluoxetine, duloxetine, paroxetine
- ATYPICAL ANTIPSYCHOTICS: olanzapine, risperidone, aripiprazole
- TRICYCLIC ANTIDEPRESSANTS: doxepin, nortriptyline, amitriptyline
GEMTESA has not been studied in concomitant use with these medications.
*Data from IQVIA PharMetrics® Plus (Pharmetrics) between November 2012 and September 2019 that evaluated adult patients taking a medication for OAB (n=838,844) and subsequently prescribed any prespecified CYP2D6 substrate (n=106).3
AE=adverse event; BP=blood pressure; BPH=benign prostatic hyperplasia; NHANES=The National Health and Nutrition Examination Survey; OAB=overactive bladder; SBP=systolic blood pressure; SNRI=serotonin-norepinephrine reuptake inhibitor; SSRI=selective serotonin reuptake inhibitor.