Always having to go can put a stop to whatever patients are doing1

Up to 40% of people aged 40+ years have OAB symptoms2
with age3
Are your patients experiencing…
- Frequency (8+ times per day) that disrupts their life?3,5
- Urge urinary incontinence or fear they may not make it to the bathroom in time?5
- A frequent, sudden rush and urgency to go?5
Many men are struggling with OAB symptoms in silence6-8
OAB and BPH prevalence increase with age1,9
OAB symptoms impact
men aged ≥60 years10
As many as
men with BPH also have OAB symptoms11-13
However, there is a significant gap in OAB treatment for male patients11
men with OAB may remain undiagnosed9
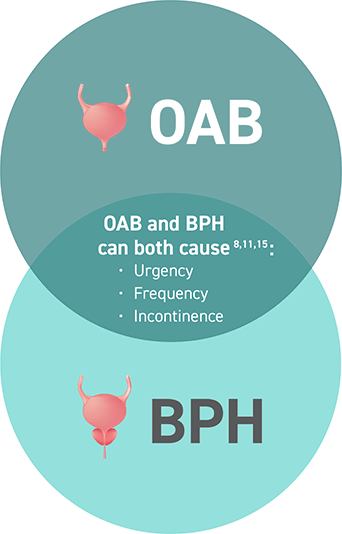
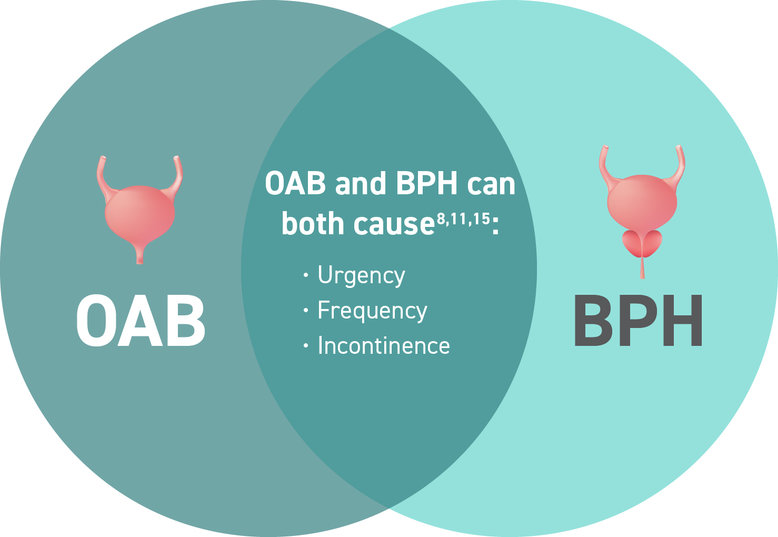
Is it BPH or OAB? Many symptoms of OAB are overshadowed by BPH11
Enduring both OAB and BPH can be confusing for patients. They may not be able to attribute which condition is causing their burdensome symptoms.11
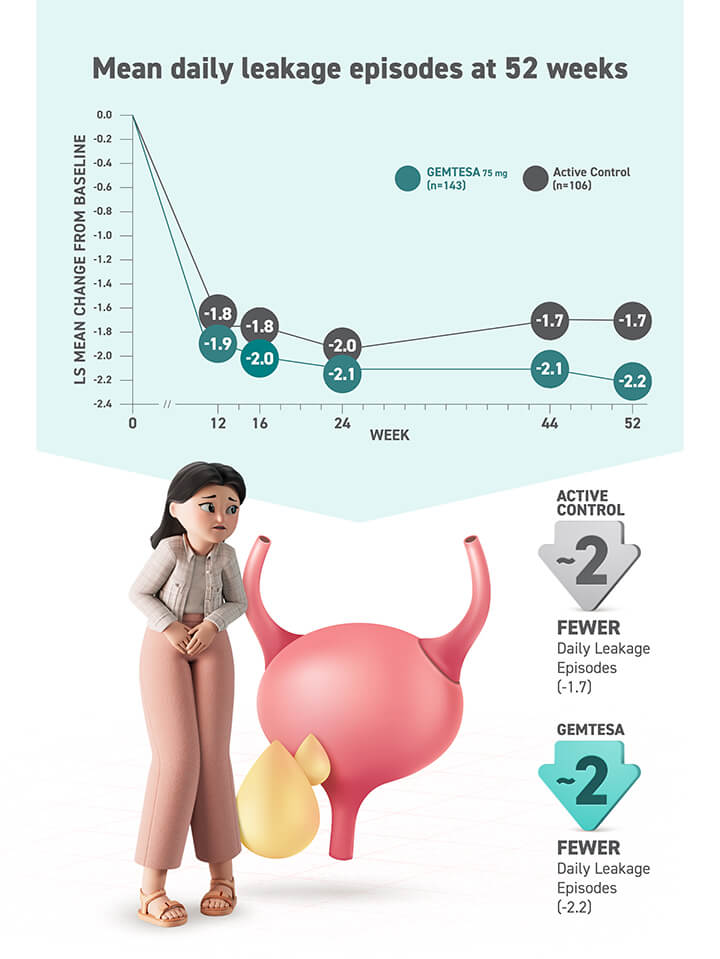
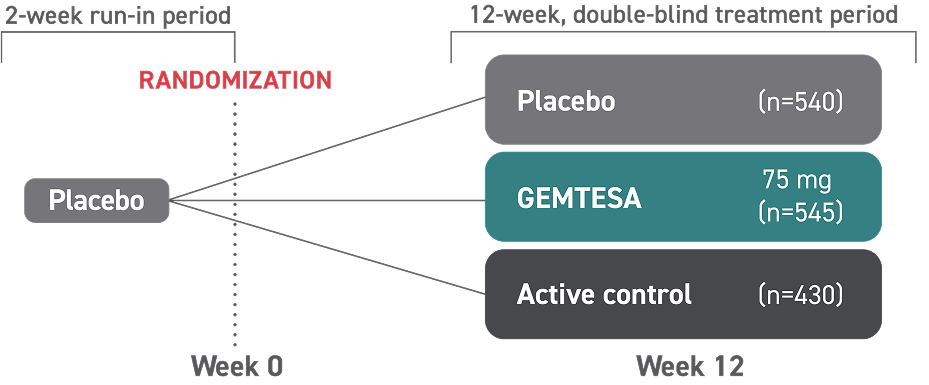
This is not an exhaustive list of all OAB symptoms. GEMTESA is only indicated to treat the 3 key symptoms of OAB.14* Treat OAB and BPH independently.