Getting candid about living
with overactive bladder (OAB)
with the

Getting candid about
living with overactive
bladder (OAB) with the 

Hear real GEMTESA Go-Getters — Holly Robinson Peete, Diane, and Dar — discuss their overactive bladder journeys. Learn how they stay on course with treatment and empower each other as fellow Go-Getters.
Holly, Diane, and Dar are real patients taking GEMTESA who have been compensated for their time.
Ready to prioritize bladder health?
Use the self-assessment tool to review your symptoms, sign up to have your results emailed to you, and receive a handy discussion guide to help you start the conversation with your doctor.
Get going with Holly and the GEMTESA Go-Getters
Transcript
“As a busy mom, actress, traveler, and advocate, my life is go, go, go. But when overactive bladder–OAB–made it so that all I could think about was, ‘I gotta go, go, go to the bathroom,' I knew I had to make a change.”
[Text on screen] Holly Robinson Peete Actor, real OAB patient, and GEMTESA spokesperson
Holly Robinson Peete is a real patient taking GEMTESA who has been compensated for her participation.
“Luckily, I listened to some of my girlfriends, my body, and, most importantly, my doctor, and found GEMTESA to help manage my OAB symptoms.”
“GEMTESA is a prescription medication for the treatment of overactive bladder in adults with symptoms of leakage episodes, urgency, and frequency.”
[Text on screen] GEMTESA is a prescription medication for the treatment of overactive bladder (OAB) in adults with symptoms of leakage episodes, urgency, and frequency.
“Do not take GEMTESA if you are allergic to vibegron or any of the ingredients in GEMTESA.”
“GEMTESA may cause serious side effects such as increasing your chances of not being able to empty your bladder.”
[Text on screen] GEMTESA may cause serious side effects such as increasing your chances of not being able to empty your bladder. Tell your doctor right away if you are unable to empty your bladder.
“My doctor told me the most common side effects may including headache, urinary tract infection, and common cold symptoms.””
[Text on screen] Most common side effects include headache, urinary tract infection, nasal congestion, sore throat or runny nose, diarrhea, nausea, and upper respiratory tract infection. Please see Important Safety Information at the end of this video.
“That was over a year ago, and now I'm making it my mission to change what ‘go, go, go,' means in my life and share my experience with others.”
“That's why I became a GEMTESA Go-Getter.”
“A Go-Getter is someone who refuses to let their lives revolve around the bathroom.”
[Text on screen] Quality time with friends
“And it's especially important to spread the word because I know so well that speaking up helps others feel seen and heard.”
“So, help me reframe the go, go, go. I hope you'll: Go — ask your doctor about GEMTESA”
[Text on screen] GO—ask your doctor about [logo] GEMTESA® (vibegron) 75 mg tablets
“Go — talk about your symptoms and explore treatment options”
[Text on screen] GO—talk about your symptoms and explore treatment options
“And Go — become a Go-Getter yourself.”
[Text on screen] Go—become a GO-GETTER yourself.
“Personally, life with GEMTESA means I am comfortable sitting further from the bathroom.”
“So come on, it's time to GO do something about OAB symptoms and become a GEMTESA Go-Getter yourself.”
[Text on screen] TIME TO GO
If you're struggling with OAB symptoms, use the self-assessment tool to start a discussion with your doctor.
Full Prescribing Information can be found at GEMTESA.com/PI
“If you're struggling with OAB symptoms, use the self-assessment tool to start a discussion with your doctor.”
[Narrator voice-over]
IMPORTANT SAFETY INFORMATION
Do not take GEMTESA® (vibegron) if you are allergic to vibegron or any of the ingredients in GEMTESA.
Before you take GEMTESA, tell your doctor about all your medical conditions, including if you have liver problems; have kidney problems; have trouble emptying your bladder or you have a weak urine stream; take medicines that contain digoxin; are pregnant or plan to become pregnant (it is not known if GEMTESA will harm your unborn baby; talk to your doctor if you are pregnant or plan to become pregnant); are breastfeeding or plan to breastfeed (it is not known if GEMTESA passes into your breast milk; talk to your doctor about the best way to feed your baby if you take GEMTESA).
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
What are the possible side effects of GEMTESA?
GEMTESA may cause serious side effects including:
- inability to empty your bladder (urinary retention). GEMTESA may increase your chances of not being able to empty your bladder, especially if you have bladder outlet obstruction or take other medicines for treatment of overactive bladder. Tell your doctor right away if you are unable to empty your bladder.
- angioedema. GEMTESA may cause an allergic reaction with swelling of the lips, face, tongue, or throat, with or without difficulty breathing. Stop using GEMTESA and tell your doctor right away.
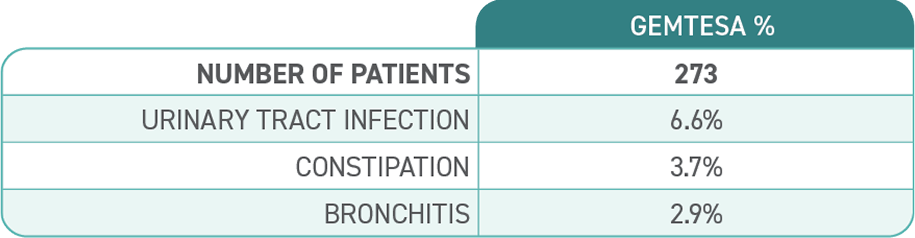
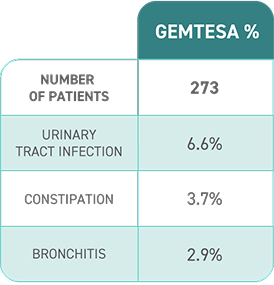
The most common side effects of GEMTESA include headache, urinary tract infection, nasal congestion, sore throat or runny nose, diarrhea, nausea and upper respiratory tract infection. These are not all the possible side effects of GEMTESA. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What is GEMTESA?
GEMTESA is a prescription medicine for adults used to treat the following symptoms due to a condition called overactive bladder:
- urge urinary incontinence: a strong need to urinate with leaking or wetting accidents
- urgency: the need to urinate right away
- frequency: urinating often
It is not known if GEMTESA is safe and effective in children.
Please see full Prescribing Information at www.GEMTESA.com/PI.
Being a GEMTESA Go‑Getter is so important to me
because I can spread awareness about OAB, rally others to care about their own bladder health, and open up the conversation. When you share stories, you never know what you might discover. I know how powerful advocacy can be—it can help others feel seen and understood.

Actor, real OAB patient, and GEMTESA spokesperson.
Living with OAB: Lessons from the GEMTESA Go-Getters
Be open and up front with your
doctor. The more they know, the
more they may be able to help you!
Actor, real OAB patient, and GEMTESA spokesperson.

Remembering to take GEMTESA daily is easy for me because I set a reminder on my phone for breakfast time. Even when I’m traveling, having that reminder never fails!
Diane is a real patient taking GEMTESA who has been compensated for her time.

I use the Let’s Go Tracker to track my progress and I take it with me to my urologist. That way we can look at the results together and see how I’m doing.
Lois is a real patient taking GEMTESA who has been compensated for her time.

OAB symptoms may worsen with age.
Mine did, and that led to some embarrassing moments. Don’t wait, speak up now.
Dar is a real patient taking GEMTESA who has been compensated for her time.

Are you a GEMTESA Go‑Getter?
By sharing your GEMTESA story, you can help break the stigma of living with OAB and inspire other patients to GO GET help. After you submit your info, you'll be contacted by one of our partners and your story may be featured here or on our social media channels!
Thank you for your interest in sharing your experience with GEMTESA for use in our promotional activities. By contacting us, you confirm that you:
Are over the age of 18
Live in the United States
Have been diagnosed with OAB
Have been prescribed GEMTESA for overactive bladder
Are currently taking GEMTESA as prescribed by your healthcare provider
Follow us:
Sign up to see if you are eligible for savings and to learn more about available resources with GEMTESA Patient Support™ (GPS).