GEMTESA may help
you score OAB
symptom relief
GEMTESA may help you score OAB symptom relief

Proven results
GEMTESA is the first and only FDA-approved medication to treat OAB (overactive bladder) in men being treated for BPH (benign prostatic hyperplasia) who experience:
- Urges so strong that they could be followed by leakage
- Unusually strong urges to urinate
- Frequent urination (8 or more times per day)

Convenient dosing
A once-daily treatment for OAB in men receiving BPH treatment.
An option if you have high blood pressure
No clinically significant changes to hypertension or increased blood pressure were seen vs placebo.
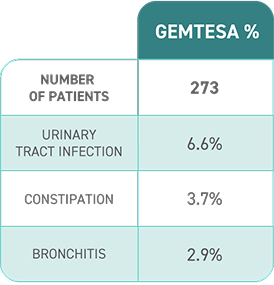
- In a study of OAB in men being treated for BPH, rates of hypertension were 9.0% with GEMTESA (50 of 553 people) vs 8.3% with placebo (46 of 551 people).
- In a separate clinical study of men and women, people taking GEMTESA and those taking placebo had similar rates of hypertension (1.7% [9 out of 545 people] vs 1.7% [9 out of 540 people], respectively) and increased blood pressure (0.7% [4 out of 545 people] vs 0.9% [5 out of 540 people], respectively).

Proven safety profile
GEMTESA has a proven safety profile and won’t interact with medications commonly taken for other conditions, including BPH treatments.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements, or if you take medications that contain digoxin.
Fiction
There's nothing I can do about bladder symptoms — I just have to “deal” with it.
Fact
While OAB is chronic and doesn’t go away on its own, it may be managed as long as you have a treatment plan. It’s not something you have to just cope with — there are treatment options like GEMTESA that may help.
Bruce made the decision to talk to his doctor about OAB when his bladder symptoms continued despite BPH treatment
Transcript
[Text on screen] Bruce’s Go-Getter Journey [Text on screen] Bruce is a real patient taking GEMTESA® (vibegron) who has been compensated for his time.
For me, it’s always great to work towards a goal — whether it’s addressing OAB or doing well in a race.
I’m Bruce, a professional runner, among many other things, like grandfather to three granddaughters, avid traveler, and unfortunately…guy who’s “gotta go” way too often! Or at least, I used to be…
[Text on screen] Please see Important Safety Information included in this video.
About 10 years ago, I knew something was off when that constant “gotta go” feeling started impacting my life — and sometimes my athletic training — a hobby I’ve had for nearly 50 years.
Health is a major priority to me, so I talked to my doctor, who diagnosed me with BPH, or benign prostatic hyperplasia — a non-cancerous enlarged prostate.
But even with treatment for BPH, I noticed I was still having bladder symptoms, especially frequency — that urge to go even when my bladder wasn’t full — and sometimes at the most inconvenient times!
For a long-distance runner, those lingering symptoms were a major burden. So, I started tracking my trips to the bathroom, and back to the doctor I went!
That's when I learned about overactive bladder — or OAB. Which has many overlapping symptoms with BPH — like urgency, frequency, and leakage episodes. It’s also chronic and can worsen if left untreated.
And you know what was news to me? Up to 75% of men with BPH have both and don’t even know it!
I can’t tell you how relieved I was to learn that it’s a real, treatable condition with non-surgical treatment options like GEMTESA — a once-daily pill that’s proven to treat OAB symptoms in men being treated for BPH.
AVO: GEMTESA is a prescription medicine used to treat the following symptoms due to a condition called overactive bladder (OAB) in adults, and in adult males taking medicine for benign prostatic hyperplasia (BPH): leakage episodes, urgency, and frequency.
It is not known if GEMTESA is safe and effective in children.
[Text on screen] GEMTESA® is a prescription medicine used to treat the following symptoms due to a condition called overactive bladder (OAB) in adults, and in adult males taking medicine for benign prostatic hyperplasia (BPH): leakage episodes, urgency, and frequency. It is not known if GEMTESA is safe and effective in children.
AVO: Do not take GEMTESA if you are allergic to vibegron or any of the ingredients in GEMTESA.
GEMTESA may cause serious side effects such as:
- Inability to empty your bladder. Tell your doctor right away if this happens.
- An allergic reaction with swelling of the lips, face, tongue, or throat, with or without difficulty breathing, and may be life-threatening. Stop using GEMTESA and get emergency medical help right away if you have any of these symptoms.
[Text on screen] Do not take GEMTESA if you are allergic to vibegron or any of the ingredients in GEMTESA.
GEMTESA may cause serious side effects such as:
- Inability to empty your bladder. Tell your doctor right away if this happens.
- An allergic reaction with swelling of the lips, face, tongue, or throat, with or without difficulty breathing, and may be life-threatening. Stop using GEMTESA and get emergency medical help right away if you have any of these symptoms.
After my doctor and I decided that GEMTESA was the right option for me, he told me about the most common side effects, including headache, urinary tract infection, nasal congestion, sore throat, or runny nose, diarrhea, nausea, and upper respiratory tract infection.
[Text on screen] Most common side effects may include headache, urinary tract infection, nasal congestion, sore throat or runny nose, diarrhea, nausea, and upper respiratory tract infection. Please see Important Safety Information at the end of this video.
And he told me that I can take GEMTESA with my BPH medication, which is important because BPH and OAB are two separate conditions that should be treated with different medicines.
For me, taking GEMTESA made perfect sense.
AVO: Tell your doctor if you’re taking medicines that contain digoxin or if you have liver or kidney problems.
[Text on screen] Tell your doctor if you’re taking medicines that contain digoxin or if you have liver or kidney problems.
Just like with my training, if I want to stay on top of my game, consistency is key. I make it a habit to take GEMTESA along with my other medications every night before bed.
Most people may see relief at 12 weeks of consistent use. Stick with it! Go the distance!
[Text on screen] Individual results may vary.
I knew GEMTESA was working for me when I was able to “go” less often and could devote more of my energy to my training and races!
Sure, I could have continued to cope with OAB by doing things like paying attention to not only how much I drank, but when I drank it, and what I drank. But GEMTESA really helped me.
So, I think it's important that you advocate for yourself when you go to your doctor and ask your doctor, “Based on what you know from your medical experience and wisdom, what do you think will work best for me?”
As the first male GEMTESA Go-Getter, it’s my goal to end the stigma around OAB and encourage men to speak up for themselves and their bladders! There is help — you just have to get the conversation going.
I tell people that they don’t have to run — just move forward.
Now get ready, set, and GO talk to your doctor today!
[Text on screen] Keep moving forward
Talk to your doctor today.
If you’re still dealing with bladder issues after BPH treatment, it may be OAB.
Full Product Information can be found at www.GEMTESA.com/PI.
[Narrator voice-over]
What is GEMTESA?
GEMTESA is a prescription medicine used to treat the following symptoms due to a condition called overactive bladder in adults, and in adult males taking medicine for benign prostatic hyperplasia (BPH):
- urge urinary incontinence: a strong need to urinate with leaking or wetting accidents
- urgency: the need to urinate right away
- frequency: urinating often
It is not known if GEMTESA is safe and effective in children.
IMPORTANT SAFETY INFORMATION
Do not take GEMTESA (vibegron) if you are allergic to vibegron or any of the ingredients in GEMTESA.
Before you take GEMTESA, tell your doctor about all your medical conditions, including if you have liver problems; have kidney problems; have trouble emptying your bladder or you have a weak urine stream; take medicines that contain digoxin; are pregnant or plan to become pregnant (it is not known if GEMTESA will harm your unborn baby; talk to your doctor if you are pregnant or plan to become pregnant); are breastfeeding or plan to breastfeed (it is not known if GEMTESA passes into your breast milk; talk to your doctor about the best way to feed your baby if you take GEMTESA).
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
What are the possible side effects of GEMTESA?
GEMTESA may cause serious side effects including:
- inability to empty your bladder (urinary retention). GEMTESA may increase your chances of not being able to empty your bladder, especially if you have bladder outlet obstruction or take other medicines for treatment of overactive bladder. Tell your doctor right away if you are unable to empty your bladder.
- angioedema. GEMTESA may cause an allergic reaction with swelling of the lips, face, tongue, or throat, with or without difficulty breathing and may be life-threatening. Stop using GEMTESA and get emergency medical help right away if you have symptoms of angioedema or trouble breathing.
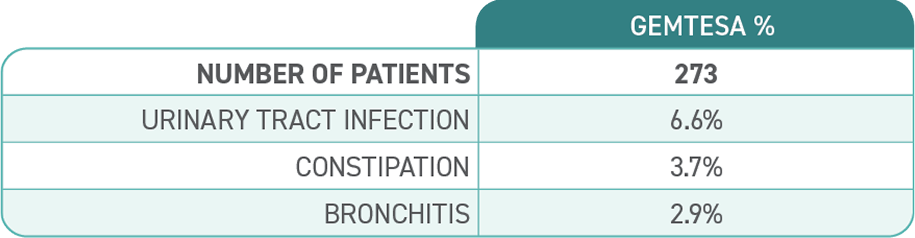
The most common side effects of GEMTESA include headache, urinary tract infection, nasal congestion, sore throat or runny nose, diarrhea, nausea and upper respiratory tract infection. These are not all the possible side effects of GEMTESA. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Transcript
[Text on screen] Bruce’s Go-Getter Journey [Text on screen] Bruce is a real patient taking GEMTESA® (vibegron) who has been compensated for his time.
For me, it’s always great to work towards a goal — whether it’s addressing OAB or doing well in a race.
I’m Bruce, a professional runner, among many other things, like grandfather to three granddaughters, avid traveler, and unfortunately…guy who’s “gotta go” way too often! Or at least, I used to be…
[Text on screen] Please see Important Safety Information included in this video.
About 10 years ago, I knew something was off when that constant “gotta go” feeling started impacting my life — and sometimes my athletic training — a hobby I’ve had for nearly 50 years.
Health is a major priority to me, so I talked to my doctor, who diagnosed me with BPH, or benign prostatic hyperplasia — a non-cancerous enlarged prostate.
But even with treatment for BPH, I noticed I was still having bladder symptoms, especially frequency — that urge to go even when my bladder wasn’t full — and sometimes at the most inconvenient times!
For a long-distance runner, those lingering symptoms were a major burden. So, I started tracking my trips to the bathroom, and back to the doctor I went!
That's when I learned about overactive bladder — or OAB. Which has many overlapping symptoms with BPH — like urgency, frequency, and leakage episodes. It’s also chronic and can worsen if left untreated.
And you know what was news to me? Up to 75% of men with BPH have both and don’t even know it!
I can’t tell you how relieved I was to learn that it’s a real, treatable condition with non-surgical treatment options like GEMTESA — a once-daily pill that’s proven to treat OAB symptoms in men being treated for BPH.
AVO: GEMTESA is a prescription medicine used to treat the following symptoms due to a condition called overactive bladder (OAB) in adults, and in adult males taking medicine for benign prostatic hyperplasia (BPH): leakage episodes, urgency, and frequency.
It is not known if GEMTESA is safe and effective in children.
[Text on screen] GEMTESA® is a prescription medicine used to treat the following symptoms due to a condition called overactive bladder (OAB) in adults, and in adult males taking medicine for benign prostatic hyperplasia (BPH): leakage episodes, urgency, and frequency. It is not known if GEMTESA is safe and effective in children.
AVO: Do not take GEMTESA if you are allergic to vibegron or any of the ingredients in GEMTESA.
GEMTESA may cause serious side effects such as:
- Inability to empty your bladder. Tell your doctor right away if this happens.
- An allergic reaction with swelling of the lips, face, tongue, or throat, with or without difficulty breathing, and may be life-threatening. Stop using GEMTESA and get emergency medical help right away if you have any of these symptoms.
[Text on screen] Do not take GEMTESA if you are allergic to vibegron or any of the ingredients in GEMTESA.
GEMTESA may cause serious side effects such as:
- Inability to empty your bladder. Tell your doctor right away if this happens.
- An allergic reaction with swelling of the lips, face, tongue, or throat, with or without difficulty breathing, and may be life-threatening. Stop using GEMTESA and get emergency medical help right away if you have any of these symptoms.
After my doctor and I decided that GEMTESA was the right option for me, he told me about the most common side effects, including headache, urinary tract infection, nasal congestion, sore throat, or runny nose, diarrhea, nausea, and upper respiratory tract infection.
[Text on screen] Most common side effects may include headache, urinary tract infection, nasal congestion, sore throat or runny nose, diarrhea, nausea, and upper respiratory tract infection. Please see Important Safety Information at the end of this video.
And he told me that I can take GEMTESA with my BPH medication, which is important because BPH and OAB are two separate conditions that should be treated with different medicines.
For me, taking GEMTESA made perfect sense.
AVO: Tell your doctor if you’re taking medicines that contain digoxin or if you have liver or kidney problems.
[Text on screen] Tell your doctor if you’re taking medicines that contain digoxin or if you have liver or kidney problems.
Just like with my training, if I want to stay on top of my game, consistency is key. I make it a habit to take GEMTESA along with my other medications every night before bed.
Most people may see relief at 12 weeks of consistent use. Stick with it! Go the distance!
[Text on screen] Individual results may vary.
I knew GEMTESA was working for me when I was able to “go” less often and could devote more of my energy to my training and races!
Sure, I could have continued to cope with OAB by doing things like paying attention to not only how much I drank, but when I drank it, and what I drank. But GEMTESA really helped me.
So, I think it's important that you advocate for yourself when you go to your doctor and ask your doctor, “Based on what you know from your medical experience and wisdom, what do you think will work best for me?”
As the first male GEMTESA Go-Getter, it’s my goal to end the stigma around OAB and encourage men to speak up for themselves and their bladders! There is help — you just have to get the conversation going.
I tell people that they don’t have to run — just move forward.
Now get ready, set, and GO talk to your doctor today!
[Text on screen] Keep moving forward
Talk to your doctor today.
If you’re still dealing with bladder issues after BPH treatment, it may be OAB.
Full Product Information can be found at www.GEMTESA.com/PI.
[Narrator voice-over]
What is GEMTESA?
GEMTESA is a prescription medicine used to treat the following symptoms due to a condition called overactive bladder in adults, and in adult males taking medicine for benign prostatic hyperplasia (BPH):
- urge urinary incontinence: a strong need to urinate with leaking or wetting accidents
- urgency: the need to urinate right away
- frequency: urinating often
It is not known if GEMTESA is safe and effective in children.
IMPORTANT SAFETY INFORMATION
Do not take GEMTESA (vibegron) if you are allergic to vibegron or any of the ingredients in GEMTESA.
Before you take GEMTESA, tell your doctor about all your medical conditions, including if you have liver problems; have kidney problems; have trouble emptying your bladder or you have a weak urine stream; take medicines that contain digoxin; are pregnant or plan to become pregnant (it is not known if GEMTESA will harm your unborn baby; talk to your doctor if you are pregnant or plan to become pregnant); are breastfeeding or plan to breastfeed (it is not known if GEMTESA passes into your breast milk; talk to your doctor about the best way to feed your baby if you take GEMTESA).
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
What are the possible side effects of GEMTESA?
GEMTESA may cause serious side effects including:
- inability to empty your bladder (urinary retention). GEMTESA may increase your chances of not being able to empty your bladder, especially if you have bladder outlet obstruction or take other medicines for treatment of overactive bladder. Tell your doctor right away if you are unable to empty your bladder.
- angioedema. GEMTESA may cause an allergic reaction with swelling of the lips, face, tongue, or throat, with or without difficulty breathing and may be life-threatening. Stop using GEMTESA and get emergency medical help right away if you have symptoms of angioedema or trouble breathing.
The most common side effects of GEMTESA include headache, urinary tract infection, nasal congestion, sore throat or runny nose, diarrhea, nausea and upper respiratory tract infection. These are not all the possible side effects of GEMTESA. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Talk to your doctor about GEMTESA — the first and only FDA-approved treatment for OAB symptoms that you can take with your BPH medication.
REMEMBER:
There’s only one GEMTESA and no generic substitute. When you fill your prescription, make sure you receive the brand name.


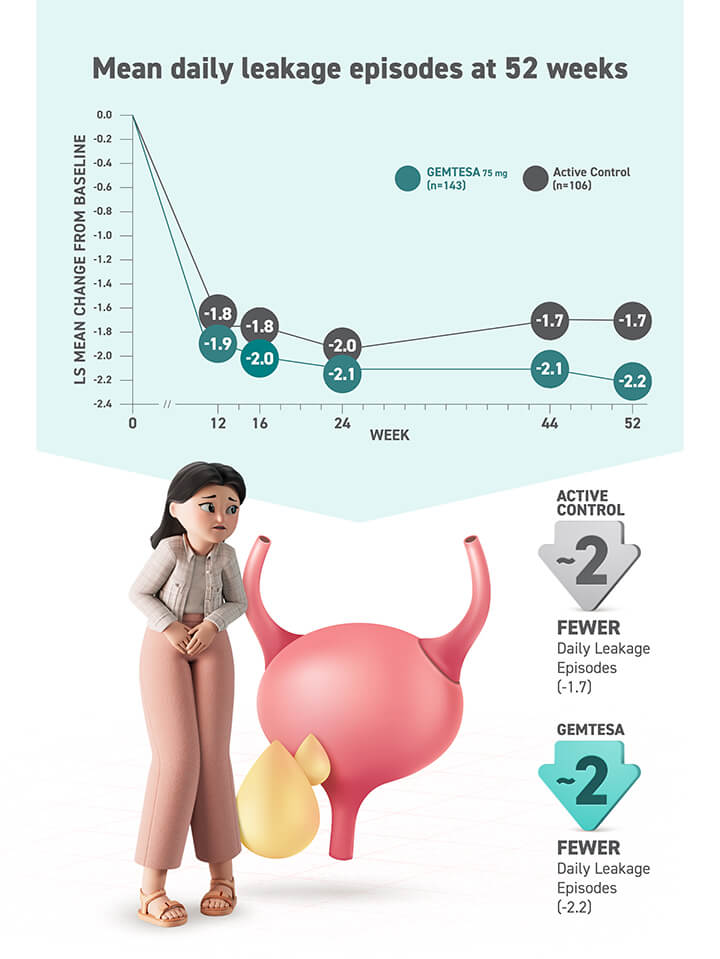
At 12 weeks, men taking GEMTESA had to go less often, had fewer “urge to go” bathroom visits, and had fewer leakage episodes
GEMTESA is the only OAB treatment proven to reduce all 3 key symptoms (urgency, frequency, and leakage) in men treated for BPH
In a clinical trial, most men with BPH taking GEMTESA saw significant reduction in all OAB symptoms at 12 weeks of consistent use as prescribed.*


fewer daily leakage episodes
An OAB treatment that may help that “can’t hold it in” feeling
- Men with BPH taking GEMTESA had about 2 (-2.19) fewer daily leakage episodes on average compared with about 1 (-1.39) fewer episode for those taking placebo†


fewer bathroom visits with
"urge to go" feelings
Stay on the go, with less urgency to go
- Men with BPH taking GEMTESA had nearly 3 (-2.88) fewer bathroom visits with “urge to go” feelings on average compared with about 2 (-1.93) fewer visits for those taking placebo‡


fewer bathroom visits
Fewer bathroom visits mean fewer daily interruptions
- Men with BPH taking GEMTESA had about 2 (-2.04) fewer bathroom visits per day on average compared with about 1 (-1.3) less visit per day for those taking placebo‡
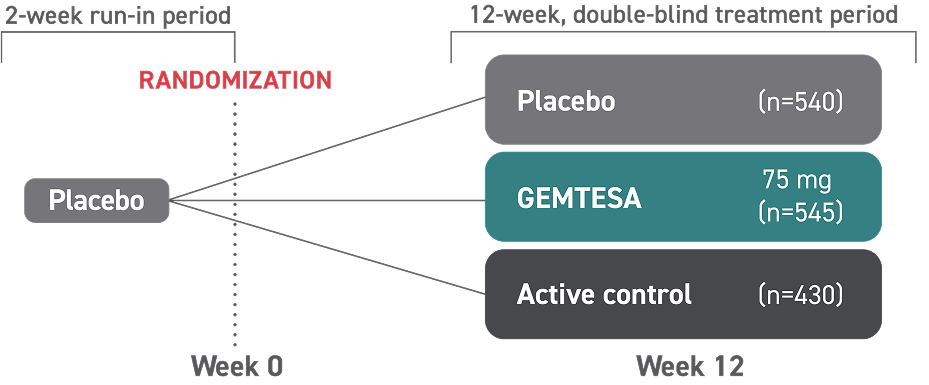
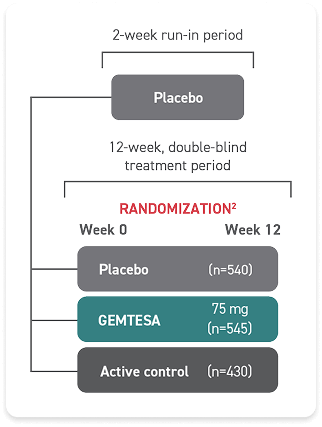
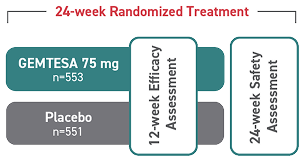
*The safety and efficacy of GEMTESA were studied in a 24-week trial in male patients with OAB being treated for BPH.
†146 people received GEMTESA and 151 people received placebo.
‡538 people received GEMTESA and 542 people received placebo.
Ready to learn more about what to expect on GEMTESA?
See Safety Profile