SUCCESSFUL MANAGEMENT OF
OAB IS CRITICAL FOR OLDER ADULTS
older adults and challenges
for caregivers

Review patient cases to learn more



LTC
* Not an actual patient.
Discover the benefits of GEMTESA for older adults with OAB

With proven efficacy and an established safety profile for older adults, GEMTESA can help reduce the impact of urinary urgency, leakage episodes, and frequency.1
No overall differences in the safety or effectiveness of GEMTESA have been observed between patients aged 65+ years and younger adult patients1
- First and only β3 agonist with proven urgency reduction data in its label
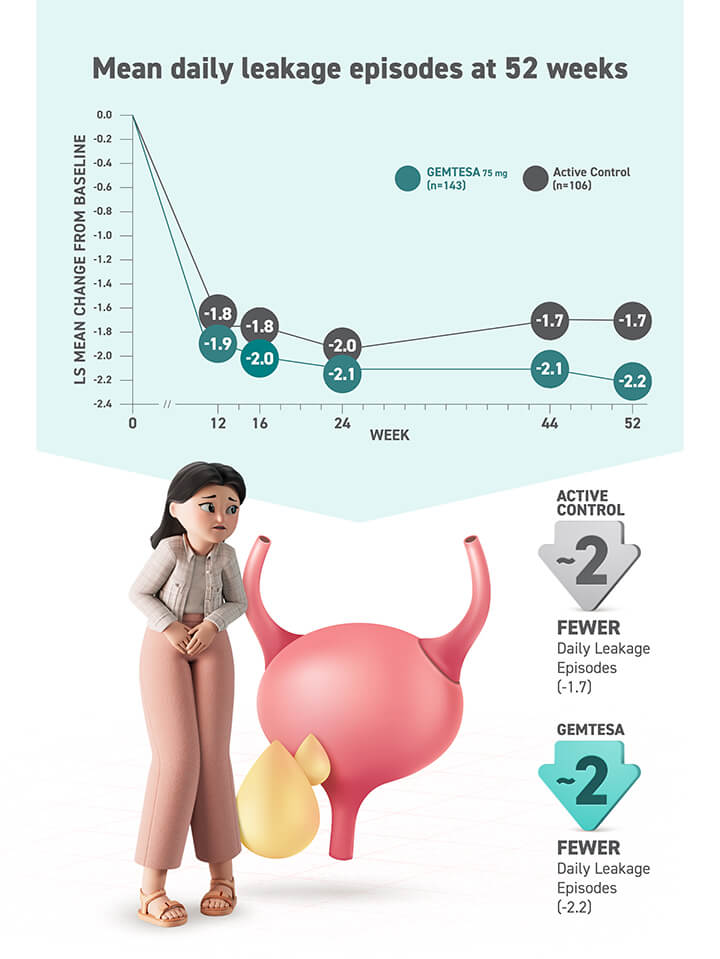
- Statistically significant reduction in all 3 key OAB symptoms* vs placebo at 12 weeks — including urgency†
- Reductions in 3 key OAB symptoms* at 12 weeks in patients aged 65+ and 75+ years
- First and only β3 agonist with a once-daily 75-mg dose with no titration, to be taken with or without food, and swallowed whole with a glass of water
- Crushability. In adults, GEMTESA tablets may be crushed, mixed with a tablespoon (~15 mL) of applesauce, and taken immediately with a glass of water
- First and only β3 agonist with no blood pressure warning in its label2‡
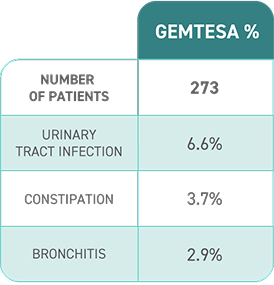
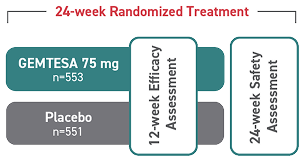
- In a 24-week study of OAB in men being treated for BPH, rates of hypertension were 9.0% with GEMTESA (n=553) vs 8.3% with placebo (n=551)
- First and only β3 agonist with no drug interaction with CYP2D6 substrates (medications commonly taken by LTC residents)2,5-8
- Concomitant use of GEMTESA increases digoxin maximal concentrations (Cmax) and systemic exposure as assessed by area under the concentration-time curve (AUC). Serum digoxin concentrations should be monitored before initiating and during therapy with GEMTESA and used for titration of the digoxin dose to obtain the desired clinical effect. Continue monitoring digoxin concentrations upon discontinuation of GEMTESA and adjust digoxin dose as needed.2
- No known association with cognitive decline, including dementia, for the β3 agonist class1
* The 3 key symptoms of OAB are urgency, micturition frequency, and urge urinary incontinence (UUI)/leakage.9
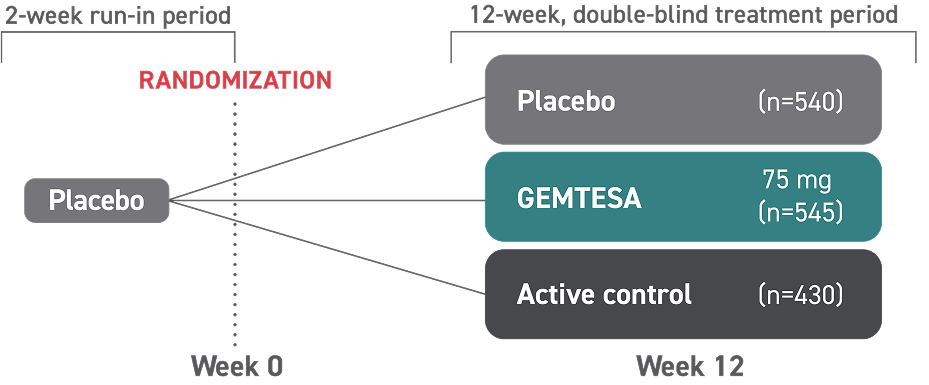
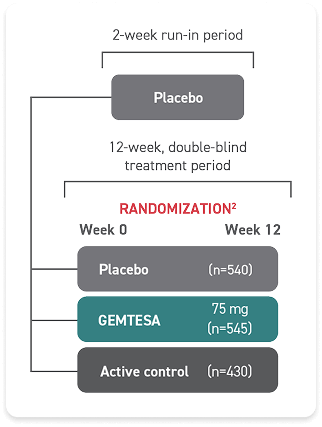
† The efficacy of GEMTESA was evaluated in a pivotal 12-week, double-blind, randomized, placebo- and active-controlled trial in patients with OAB (UUI, urgency, and urinary frequency). For study entry, patients had to have symptoms of OAB for at least 3 months, with an average of 8 or more micturitions per day and at least 1 UUI per day, or an average of 8 or more micturitions per day and an average of at least 3 urgency episodes per day. A total of 1515 patients received at least 1 daily dose of placebo (n=540), GEMTESA 75 mg (n=545), or an active-control treatment (n=430). The majority of patients were Caucasian (78%) and female (85%) with a mean age of 60 (range 18 to 93) years.2
‡ In a 4-week, randomized, placebo-controlled, ambulatory blood pressure study in OAB patients (n=200), daily treatment with GEMTESA 75 mg was not associated with clinically significant changes in blood pressure. Subjects enrolled in this study had a mean age of 59 years and 75% were female. Thirty-five percent of subjects had preexisting hypertension at baseline and 29% of all subjects were taking at least 1 concomitant antihypertensive medication.5
BPH=benign prostatic hyperplasia; LTC=long-term care; OAB=overactive bladder; UTI=urinary tract infection.