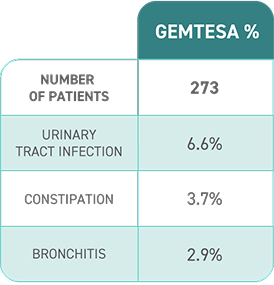
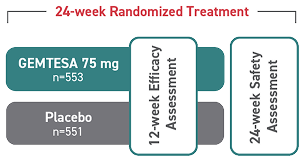
OAB in long-term care

Urinary incontinence (UI) may be a symptom of a medical condition called OAB and may affect about 70% of LTC residents in the US1,2
Early identification and diagnosis may help residents affected by burdensome OAB symptoms2,5
Symptom recognition is a critical first step toward OAB care. As many residents suffer from cognitive decline, they may have difficulty reporting OAB symptoms. Here's what to look for3,6-8:
Urgency (the hallmark symptom)
- Sudden, strong urge to urinate immediately
Urge urinary incontinence (UUI)/leakage
- Involuntary leakage episodes (which may require use of absorbent products)
Frequency
- Urinating 8+ times per day
* Based on a study of 71 DONs who completed a 30-minute online survey conducted from February 27, 2020 to May 11, 2020.3
† Cross-sectional retrospective analysis of 175,632 nursing facility residents from October 1, 2010 to September 30, 2012.10
‡ From an IQVIA database reporting baseline characteristics of 159,785 LTC patients with OAB.9
UI=involuntary leakage of urine; LTC=long-term care; OAB=overactive bladder.