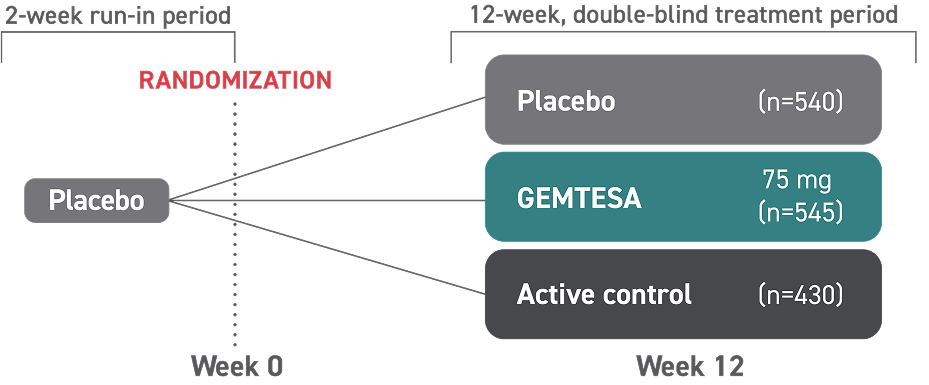
ONLY GEMTESA IS PROVEN TO TREAT OAB SYMPTOMS* IN MEN BEING PHARMACOLOGICALLY TREATED FOR BPH1,2

Help male patients strike against all 3 key symptoms* of OAB1,2
Do urinary-related issues persist for your patients being treated for BPH? Make a difference for your patients — add GEMTESA to a BPH treatment regimen to help reduce OAB symptoms.
*OAB symptoms include urgency, frequency, and UUI.4
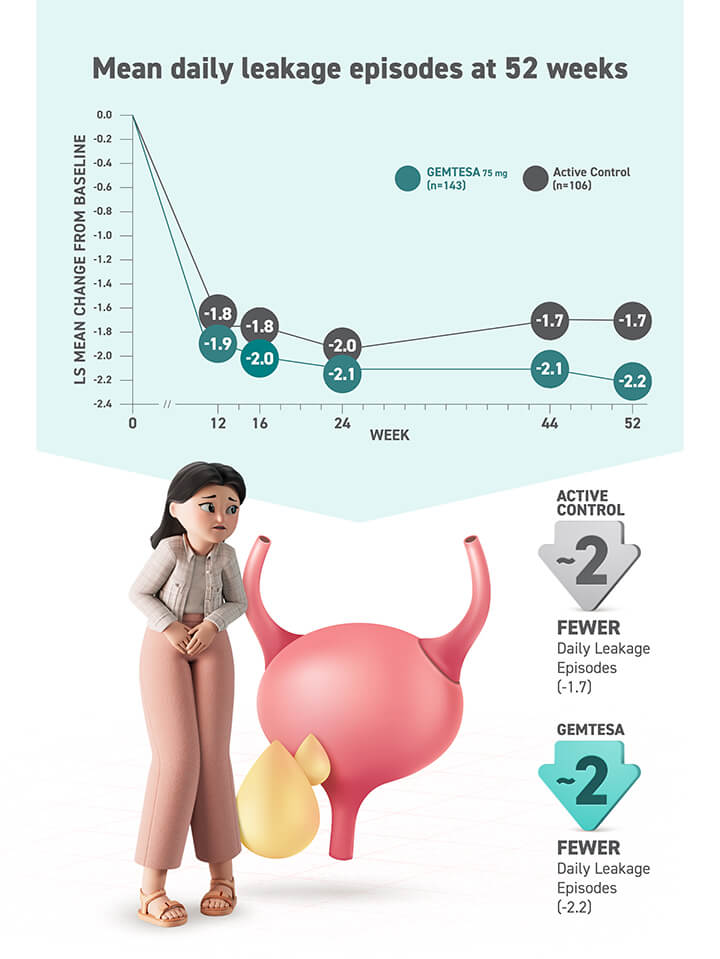
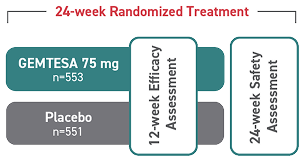
Relief from urgency is within reach2
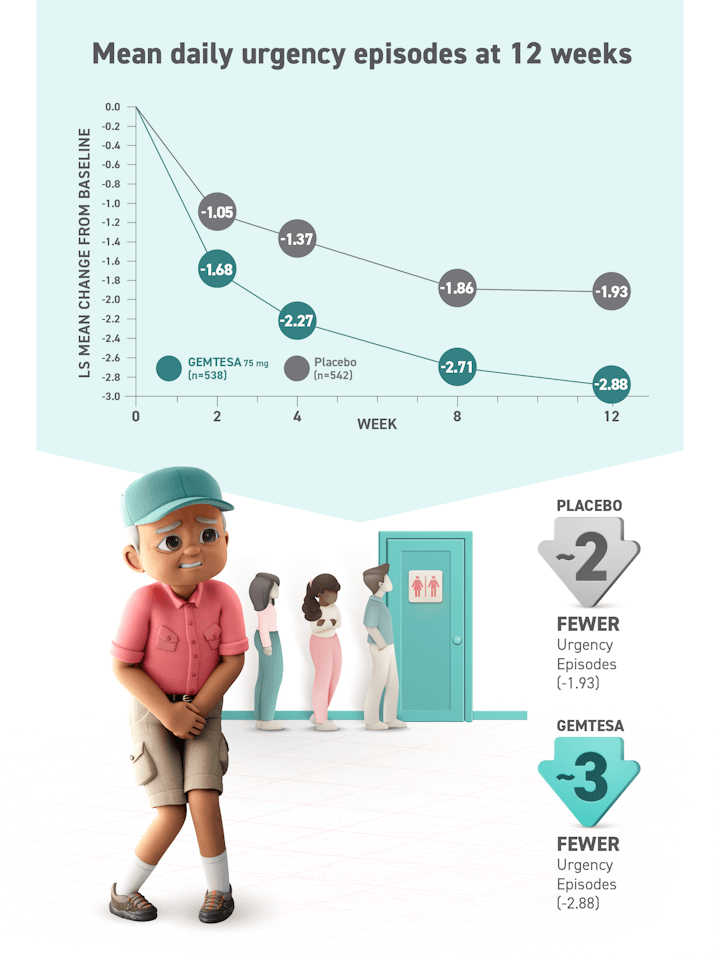
LS=least squares; SE=standard error; UUI=urge urinary incontinence.
LS=least squares; UUI=urge urinary incontinence.
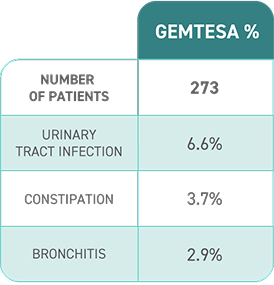
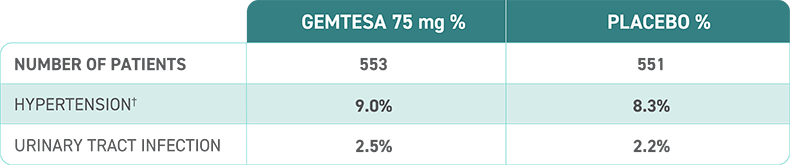
Proven safety profile in male patients being treated for BPH1,2
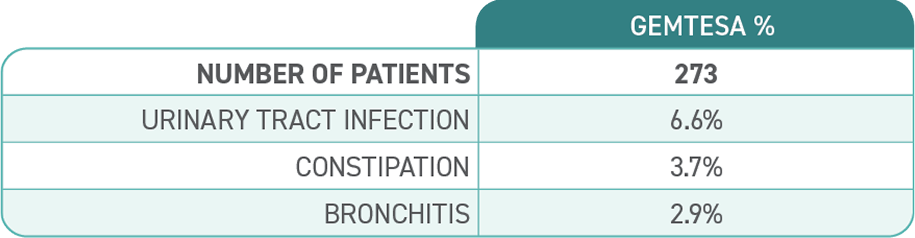
Adverse reactions, exceeding placebo rate, reported in ≥2% of patients treated with GEMTESA 75 mg for up to 24 weeks

97.1%
OF PATIENTS TAKING GEMTESA completed the study without discontinuing treatment due to AEs2
GEMTESA was also evaluated for long-term safety in a 28-week extension study in 276 patients who completed the 24-week study. Of the 276 patients who received GEMTESA 75 mg once daily in the extension study, 124 patients were treated for a total of 1 year. There were no additional adverse reactions reported that were not already reported in the pivotal and extension studies.1
† Defined as an average systolic blood pressure (SBP) ≥140 mm Hg or diastolic BP (DBP) ≥90 mm Hg on 3 assessments at two consecutive visits, in non-hypertensive patients.1
† Defined as an average increase of SBP ≥20 mm Hg or DBP ≥10 mm Hg on 3 assessments at two consecutive visits, or the initiation or increase in dose of antihypertensive medications at any visit, in hypertensive patients.1
GEMTESA is the only β3 treatment with no drug interaction with commonly prescribed CYP2D6-metabolized agents, including tamsulosin1,3
Concomitant use of GEMTESA increases digoxin maximal concentrations (Cmax) and systemic exposure as assessed by area under the concentration-time curve (AUC). Serum digoxin concentrations should be monitored before initiating and during therapy with GEMTESA and used for titration of the digoxin dose to obtain the desired clinical effect. Continue monitoring digoxin concentrations upon discontinuation of GEMTESA and adjust digoxin dose as needed.1
Make GEMTESA your first choice for effective OAB management in men — regardless of if they are being treated for BPH1,2
AE=adverse event; BPH=benign prostatic hyperplasia; OAB=overactive bladder.